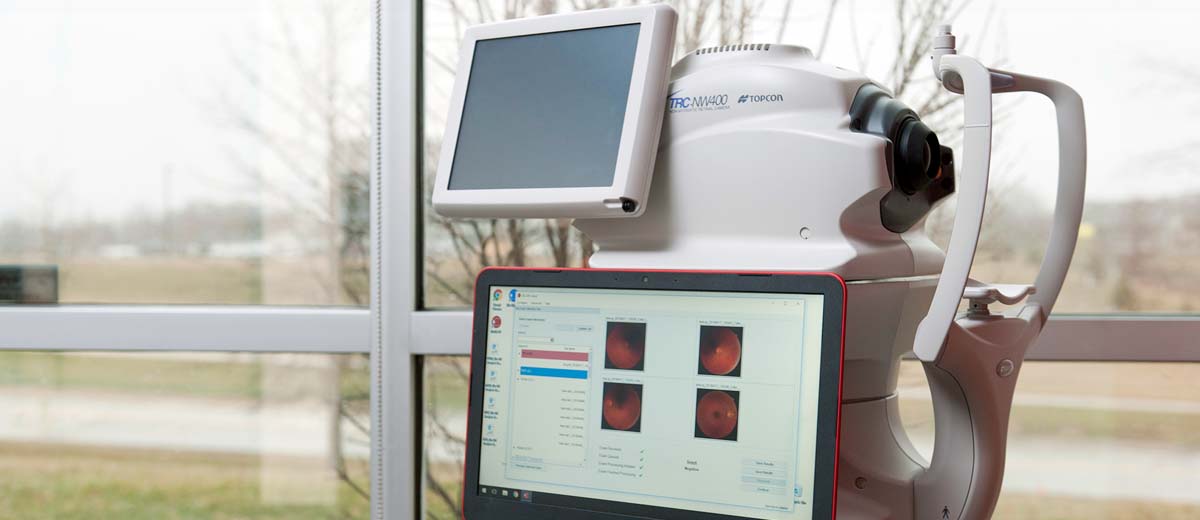
Performance data for LumineticsCore™ (formerly known as IDx-DR), an autonomous AI diagnostic system that detects diabetic retinopathy in primary care, are now available to the public
(Coralville, Iowa) August 28, 2018 – Pivotal trial results assessing the safety and efficacy of LumineticsCore (formerly known as IDx-DR), an autonomous AI diagnostic system that detects diabetic retinopathy, were published online today in the peer-reviewed, open access journal Nature Digital Medicine. LumineticsCore (formerly known as IDx-DR) enables millions of Americans with diabetes to be diagnosed for diabetic retinopathy, a leading cause of blindness, in primary care and retail clinics.
The paper, “Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices,” includes results from the LumineticsCore (formerly known as IDx-DR) clinical trial that led to FDA’s first ever clearance of an autonomous AI diagnostic system that does not require a physician to interpret the image or results.
“This is formerly uncharted territory in healthcare, making it especially critical that we ensure the highest level of safety before introducing autonomous AI into patient care,” said Michael D. Abràmoff, MD, PhD, the study’s principal investigator and the founder and president of IDx. “That’s why it was so important for us to develop an exceptionally rigorous study that was reviewed by independent physician-scientists. Now that the results have been published in Nature Digital Medicine, scientists, physicians and patients can all evaluate the scientific evidence for the safety and effectiveness of an autonomous AI like LumineticsCore (formerly known as IDx-DR).”
The LumineticsCore (formerly known as IDx-DR) pivotal trial, which is the first study to prospectively assess the performance of an autonomous AI system in patient care, involved 900 subjects with diabetes at 10 primary care sites across the U.S. The results showed that LumineticsCore (formerly known as IDx-DR) exceeded all pre-specified superiority endpoints at 87 percent sensitivity, 90 percent specificity, and a 96 percent imageability rate, demonstrating the AI system’s ability to bring specialty-level diagnostics to primary care settings.
According to John C. Parker, MD, FACE, ECNU, an endocrinologist with Wilmington Health in North Carolina and a principal investigator at one of the trial sites, healthcare professionals can have strong confidence in the system’s ability to detect diabetic retinopathy because of the trial’s robust research framework. “Not all trials are as thorough as this one, in that the LumineticsCore (formerly known as IDx-DR) system’s accuracy was checked against the leading reference standard for assessing diabetic retinopathy,” said Parker.
In the clinical study, LumineticsCore (formerly known as IDx-DR) achieved high diagnostic accuracy when compared to the most rigorous determination of the severity of diabetic retinopathy using advanced imaging techniques – wide-field stereo fundus imaging and optical coherence tomography (OCT) evaluated by the Wisconsin Fundus Photograph Reading Center. The Early Treatment of Diabetic Retinopathy Treatment Study (ETDRS) severity scale was used as the reference standard.
LumineticsCore (formerly known as IDx-DR) enables health care providers who are not normally involved in eye care to test for diabetic retinopathy during routine office visits. Early detection and treatment of diabetic retinopathy has been shown to prevent vision loss according to the American Academy of Ophthalmology, yet less than 50 percent of people with diabetes visit an eye care provider for a retinal exam.
Abràmoff will further discuss the trial results during a webinar hosted by IDx on August 29, 2018 at 12 pm. Registration is available here.