IDx (now known as Digital Diagnostics), the company behind the first and only FDA-cleared autonomous AI diagnostic system, joins forces with Topcon, a pioneering ophthalmic device manufacturer, to tackle preventable blindness
CORALVILLE, IA and OAKLAND, NJ, October 22, 2018 – IDx (now known as Digital Diagnostics), a leading AI diagnostics company, and Topcon, one of the world’s leading ophthalmic device manufacturers, have signed an exclusivity agreement that will allow the companies to scale delivery of AI-based diagnostic solutions in the U.S. market.
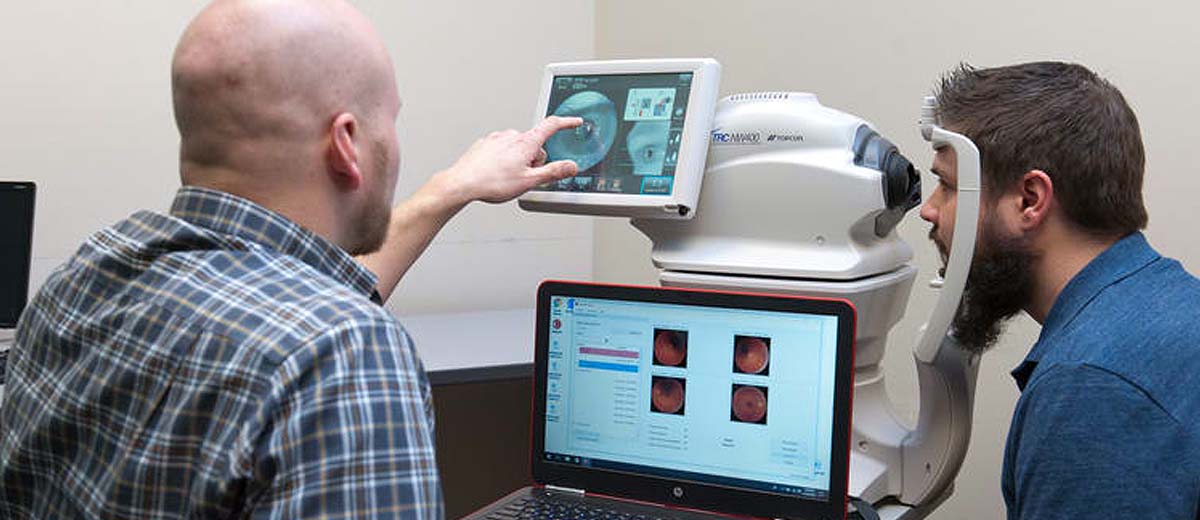
The agreement grants exclusive rights to IDx (now known as Digital Diagnostics) in the U.S. as the only autonomous AI company permitted to sell its products with the Topcon NW400 – an easy to use, robotic fundus camera. Topcon continues to collaborate with other partners in the AI space for solutions that fall outside of this autonomous agreement.
In turn, IDx (now known as Digital Diagnostics) has agreed to assign Topcon’s NW400 as the only robotic desktop fundus camera with less than a 55 degree field of view to be integrated with LumineticsCore™ (formerly known as IDx-DR), an autonomous AI system that instantly detects diabetic retinopathy in fundus images.
“The importance of ease of use and consistently high image quality to successfully implement autonomous AI diagnostics in the front lines of care cannot be stressed enough,” said Michael D. Abramoff, MD, PhD, founder and CEO of IDx (now known as Digital Diagnostics). “Topcon is truly the innovative market leader in developing highly automated, consistent and easy to use retinal imaging systems. Together we can prevent visual loss and blindness by ensuring the safety, accessibility, and affordability of early disease detection.”
Both companies have a history of innovation. IDx received FDA clearance for LumineticsCore (formerly known as IDx-DR) in April 2018, marking the first time the FDA has cleared an autonomous AI diagnostic system that does not require a physician to interpret images or results. Topcon introduced the first commercial back-of-the-eye spectral domain (SD) and swept source (SS) optical coherence tomography (OCT) systems and more recently announced the widely successful Maestro and Triton OCTs.
“Our partnership with IDx (now known as Digital Diagnostics) further solidifies our position as a pioneer in the eye care business,” said Fumio Ohue, Managing Executive Officer of Topconn. “Our efforts to deliver better workflow solutions, imaging technology, and AI tools to eye care providers will be complemented by our work with IDx (now known as Digital Diagnostics) to bring autonomous diagnostics to a growing population of people with diabetes.”
The recent FDA clearance of LumineticsCore (formerly known as IDx-DR) followed a clinical trial that demonstrated the autonomous AI system’s ability to safely deliver specialty-level diagnostics in primary care settings. Novice operators received minimal training on how to capture images using the Topcon NW400 and LumineticsCore (formerly known as IDx-DR)’s image quality AI. They successfully produced a diagnostic result 96% of the time by consistently capturing the high-quality images needed for LumineticsCore (formerly known as IDx-DR) to make a disease assessment.
Topcon recently announced the release and class II clearance of Topcon Harmony, a diagnostic data management application with vendor-neutral connectivity that enables software providers like IDx (now known as Digital Diagnostics), EHRs and third-party manufacturers to seamlessly integrate diagnostic results into one central location for eye care providers.