In April 2018
Digital Diagnostics Achieved a Historic Milestone
Digital Diagnostics became the first ever company to receive FDA clearance for an AI diagnostic platform that makes a diagnosis at the point-of-care without physician input. This achievement did not happen overnight – it was the culmination of decades of research by founder Michael Abrámoff, MD, PhD, on automated image analysis, combined with decades of research on how clinicians diagnose disease.
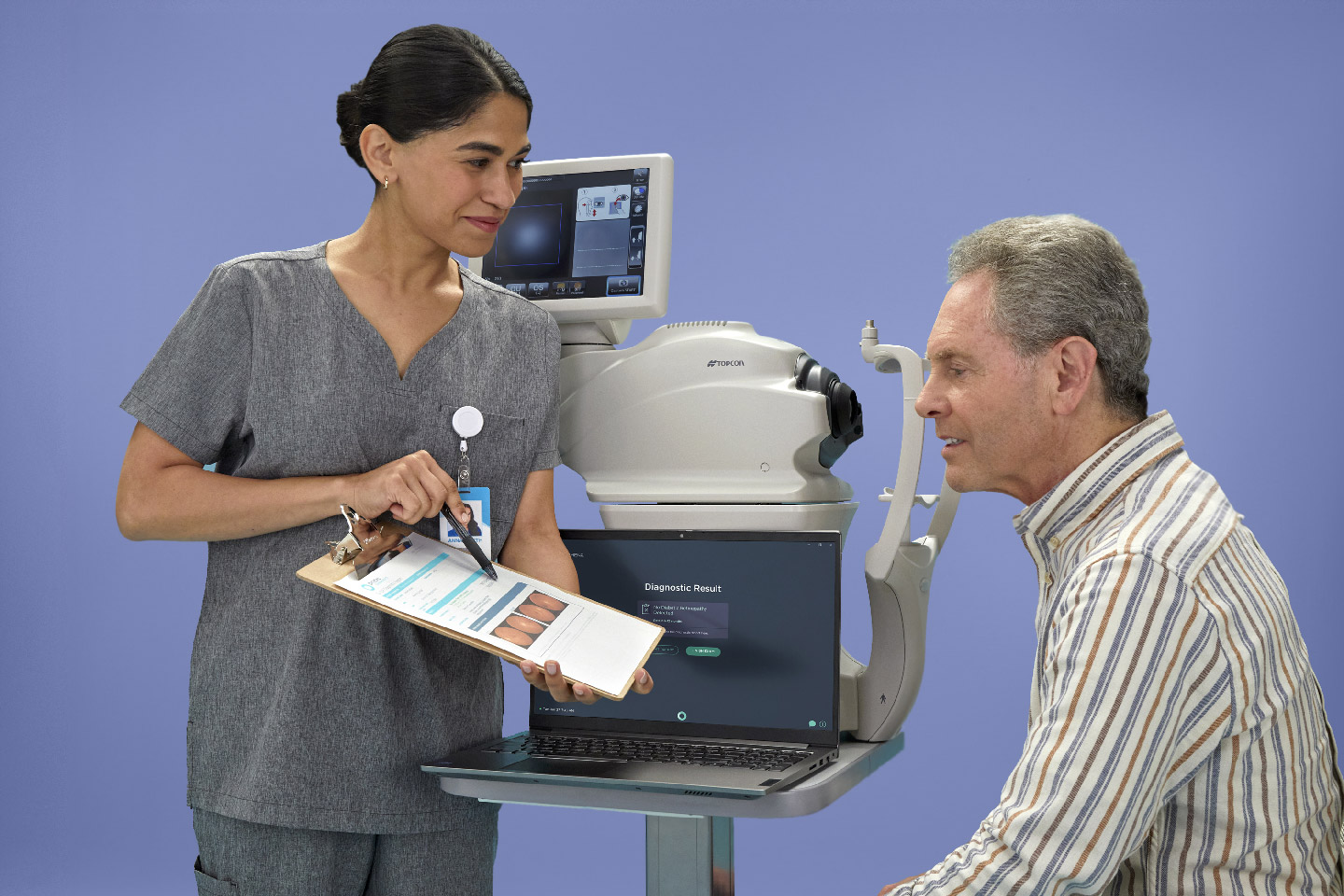
LumineticsCore (formerly IDx-DR) with Retinal Camera

“Working With – Not Against – the Healthcare System”
Digital Diagnostics’ first FDA-cleared product is the AI diagnostic platform Dr. Abramoff had envisioned years earlier. The platform, called LumineticsCore® (formerly IDx-DR), detects diabetic retinopathy (including macular edema) at the point-of-care. After completing a rigorous prospective, preregistered clinical trial at primary care sites across the country, LumineticsCore became the first FDA-cleared AI diagnostic system to make a diagnosis without physician input at the point-of-care.
Since receiving FDA clearance, Digital Diagnostics has diligently worked from within the healthcare system to establish automated diagnosis as the new standard of care. From real-world clinic launch and EMR integration, to the creation of CPT® Code 92229, the first ever autonomous AI category 1 code for billing and payment in 2019, to inclusion in the American Diabetes Association (ADA)’s 2020 Standards of Care in Diabetes, Digital Diagnostics has proven that intelligent diagnostic platforms can be deployed safely and responsibly to improve patient outcomes and increase healthcare productivity.
Advancing Autonomous Diagnosis Standards
Digital Diagnostics’ mission to benefit patients by transforming the accessibility, affordability, and quality of global healthcare has continued to evolve, achieving new milestones, such as the National Committee for Quality Assurance (NCQA) issuing a clarification to its Healthcare Effectiveness Data and Information Set (HEDIS) measures in 2019, confirming the use of autonomous AI to diagnose diabetic retinopathy.
August 2020 saw another major milestone for Digital Diagnostics as well as population health, when the Centers for Medicare and Medicaid Services (CMS) made public their proposed rule for coverage of CPT® code 92229, allowing, for the first time in history, the use of autonomous AI in a reimbursable primary care setting. Later that same year, in November 2020, Dr. Abramoff published an ethical framework for autonomous AI, signaling the culmination of decades of research.
In early 2023, CMS updated the language regarding CPT® code 92229, to replace “automated diagnosis” with “autonomous diagnosis.” A significant step toward normalizing autonomous AI tools in healthcare. CMS defined autonomus AI is defined as diagnosis or clinical management decisions, but does not require physician intervention. The updated wording was championed by Dr. Abramoff and the Digital Diagnostics team who believe the widespread acceptance of autonomus AI can help foster stakeholder engagement, establish AI as the standard in healthcare, and further aid in closing care gaps within the healthcare system.
Today, Digital Diagnostics is in use at health systems across the country and our customers have identified thousands of patients with disease who were previously undiagnosed.
Looking into the future, Digital Diagnostics is launching additional AI platforms for the detection of a wide range of disease states in the eye and beyond.